
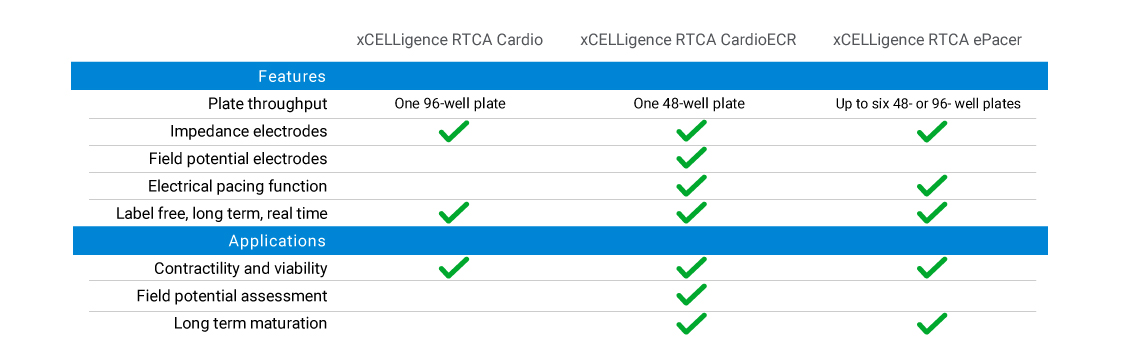
The Agilent xCELLigence RTCA Cardio instrument portfolio provides researchers with solutions for safety/toxicity assessment, cardiac drug discovery, cardiac disease model research, and other applications. The portfolio features instruments with various levels of throughput to meet your research needs and can all be used in a standard tissue culture incubator.
The full-featured CardioECR instrument combines high frequency measurement of cell-induced electrical impedance with multi-electrode array (MEA) technology to simultaneously assess cardiomyocyte contractility, viability, and electrophysiology. The CardioECR and ePacer instruments can also be used to functionally mature hiPSC cardiomyocytes with its electronic pacing function.

Impedance biosensors to measure contractility and viability.

Impedance biosensors and MEA technology to measure contractility, viability, and field potential.

High throughput instrument for electrical pacing of hiPSC-cardiomyocytes and contractility and viability measurements using impedance.

Human induced pluripotent stem cell cardiomyocytes (hiPSC-CM) have been used to assess drug liability. The xCELLigence RTCA CardioECR system offers a unique multiplex detection method to probe and understand the underlying toxicity of compounds and pharmaceuticals.

The pathophysiological cellular phenotypes of genetically heritable heart diseases can be modeled using disease-specific human induced pluripotent stem cell cardiomyocyte (hiPSC-CM). Modeling enables broader understanding of the mechanisms leading to compromised electrical and contractile coupling.
Long-term electrical pacing is quickly becoming the preferred method of choice to improve the maturation status of hiPSC-CMs in a consistent and scalable manner. Improve gene expression, protein expression, and contractile response in hiPSC-CMs for drug safety testing, cardio drug discovery, and disease modeling.

Human induced pluripotent stem cell cardiomyocytes (hiPSC-CM) offer an exciting new model system to test both the efficacy and toxicity of new therapeutic approaches, including inotropic compounds that serve to modulate the force of cardiomyocyte contractility.

Driven by HESI, FDA and Safety Pharmacology Society, the CiPA initiative aims to evaluate potential modifications to current regulatory FDA guidelines for cardiac safety assessment. The xCELLigence RTCA CardioECR was selected as a core technology for validation and has participated in Phase I and II.
For Research Use Only. Not for use in diagnostic procedure.
RA44375.5063425926
Please fill out the form to request a quote or to be contacted by an Agilent representative.