Transform Your Pharmaceutical End Product Testing
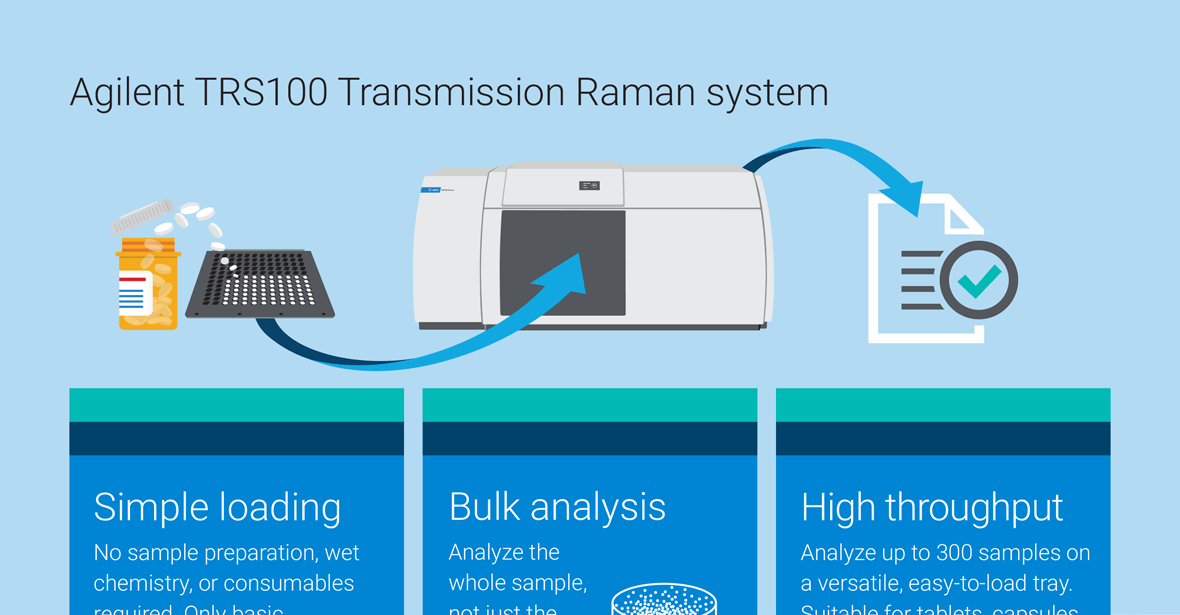
Agilent TRS100 Raman quantitative pharmaceutical analysis system
Are you spending days testing drug batches for content uniformity?
Eliminate sample preparation and reduce QC testing by days—or weeks—with transmission Raman spectroscopy (TRS).
TRS is ideal for quantitative measurements of oral solid dose forms, such as tablets and capsules. The technique complements HPLC, UV-Vis, and other traditional analytical techniques for content uniformity (CU) testing, and offers these advantages:
- No sample preparation or solvents required
- Nondestructive and takes seconds per measurement
- Ensures that the whole sample stays intact and gathers information about active ingredients within the bulk of the sample

Improve analytical workflows with TRS: On-demand webinar
Anders Sparén, associate principal scientist at AstraZeneca, talks about how TRS can be used to improve analytical workflows and operational efficiencies. In this webinar, you will:
- Explore different approaches for interpreting and evaluating spectral data.
- Learn how to apply the benefits of TRS toward an enhanced control strategy.
- Find out how rapid, nondestructive technology can benefit your lab.
Discover how TRS can augment your QC workflow
Concerned about compliance? Don’t be.
The Agilent TRS100 has features that facilitate working in compliant environments. It is ideal for labs that must comply with US FDA 21 CFR Part 11, as well as USP and EP chapters for Raman spectroscopy.
Pharmaceutical manufacturing facilities have also found that switching from standard methods to TRS has:
- Reduced release testing for an 8-batch campaign from 28 hours to 2 hours
- Saved $160,000 over one year with over 2,000 batches released
- Enabled significant testing scale-up without a corresponding increase in personnel or instrument resources
- Allowed testing to be moved into the manufacturing area—increasing efficiency by freeing the QC lab for more specialized tasks


Make a smart, sustainable choice
The Agilent TRS100 has been recognized by the independent third party My Green Lab for its sustainable attributes and has been assigned an accountability, consistency, and transparency (ACT) label based on its environmental impact.
Learn moreTRS application notes
The fundamentals of TRS
Raman spectroscopy, a vibrational spectroscopic technique, provides a chemical fingerprint of the compounds being analyzed. The spectra produced have sharp, distinctive peaks that correspond to molecular vibrations of the chemical functional groups.
TRS is a measurement that scatters light through a material, and is well suited for pharmaceutical tablets and capsules. The laser is on the opposite side of the sample to the detector, resulting in the Raman signal being collected from the bulk of the material. In pharmaceutical products, each ingredient can have many Raman peaks that combine in number and intensity to provide the spectrum.
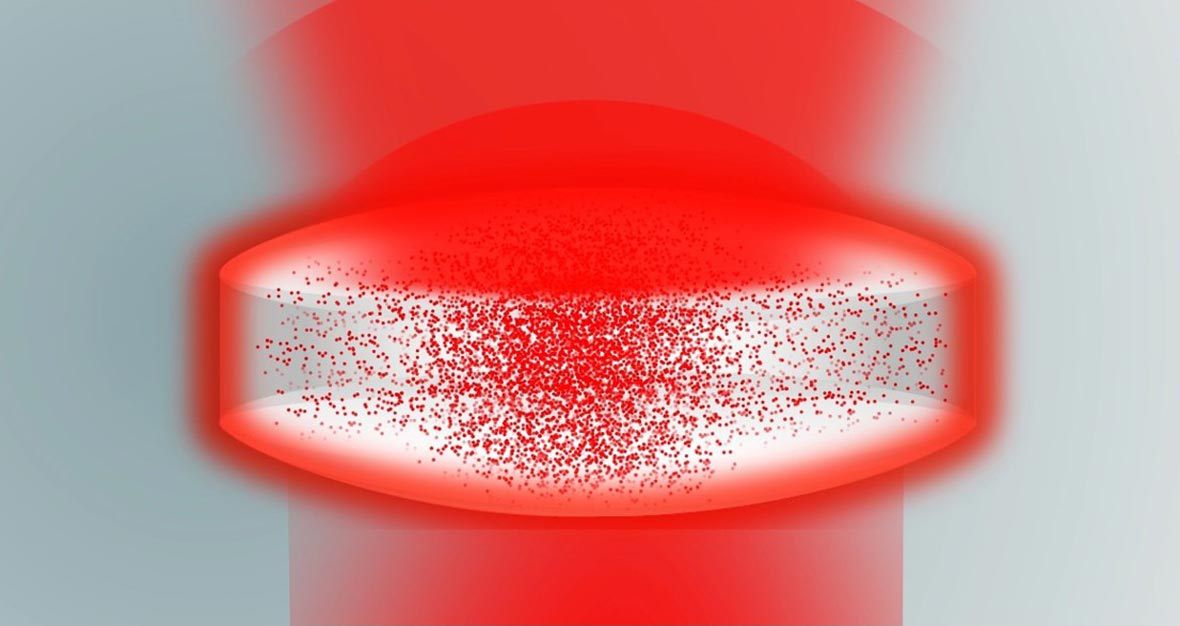
For a closer look at TRS technology, watch our educational video series
How much could your lab save with TRS?
Adding Transmission Raman spectroscopy as a complimentary content uniformity (CU) analytical method could make a big difference in your bottom line.
To see how your savings can add up, input the key variables of your existing CU analytical workflows into this quick calculator tool.
| Input your variables | Input | |
|---|---|---|
| batches | ||
| hours | ||
| mL | ||
| minutes |
| Compare the results | Traditional analysis | TRS | |
|---|---|---|---|
| Cost per batch | $55 | ||
| Total workflow time | 1 hr | ||
| Total solvent used | 0 L | ||
| Cost per sample | $5 | ||
Estimated annual cost savings: per year
How does the Agilent TRS100 make these savings possible?
TRS provides a fast, nondestructive bulk analysis of tablets and capsules. This regulatory-approved technique negates the need for sample preparation, saving you time, solvents, and consumables.
Avoid sample preparation
Save operator hours per year that could be used elsewhere.
saved per year.
Analyze without solvents
Save L of solvent per year by eliminating sample preparation, clean up, and disposal.
saved per year.
Speed up CU testing
times faster than traditional analysis.
saved per year.
Savings increase when you implement multiple analytical methods
When multiple analytical methods are deployed, your cost savings and return on investment increase dramatically.
The calculations shown on this page are illustrative estimations using industry standard values verified with existing system users. To work through a customized calculation with your local Transmission Raman product specialist, contact us.
Request a custom calculationHear how pharmaceutical labs are advancing their research with TRS
What are the advantages of Raman spectroscopy? How does Raman spectroscopy compare with other traditional techniques? What technologies should your lab focus on in the face of ever-tightening budgets?
You’ll learn the answers to these and other questions in our new webinar, featuring a panel of thought leaders from the pharmaceutical industry.
- Understand the rationale and business case of TRS, as well as the key steps for method development success.
- Hear how companies are overcoming the lack of education about TRS.
- Explore how regulatory concerns apply to innovator pharmaceutical corporations versus manufacturers of generics.
- Listen to stories about hands-on implementation of TRS in pharmaceutical manufacturing, and the challenges faced during this process.