Ready to optimize your peptide workflow?
Get in touch with one of our specialists to find the right solution for your needs.
Peptide therapeutics are transforming the pharmaceutical landscape—from treating metabolic disorders to revolutionizing obesity care. As this field grows, so does the need for precise, reliable analytical tools.
At Agilent, we specialize in helping scientists like you achieve high-resolution peptide analysis and purification. Our portfolio of reversed-phase HPLC and UHPLC columns is engineered to deliver accuracy, speed, and scalability—whether you're analyzing synthetic peptides or scaling up for preparative purification.
Why Agilent?
Who do we help?
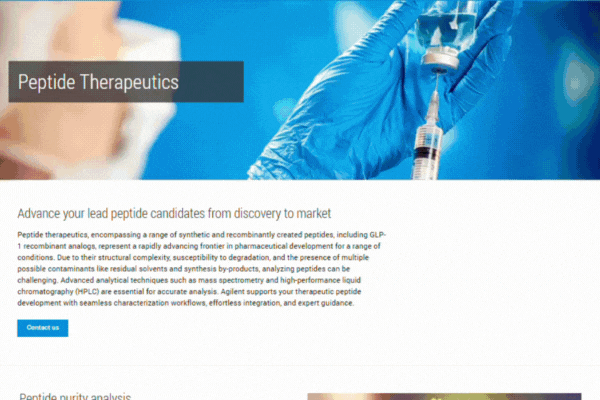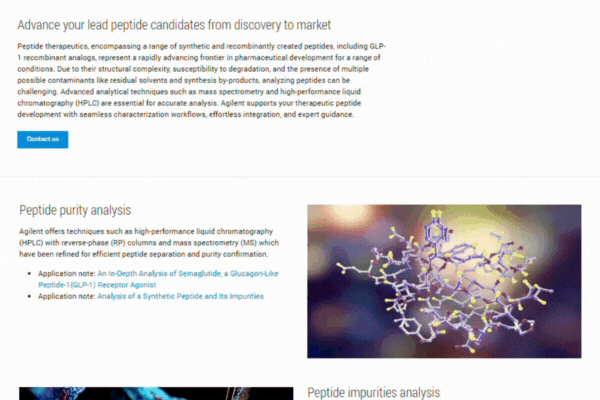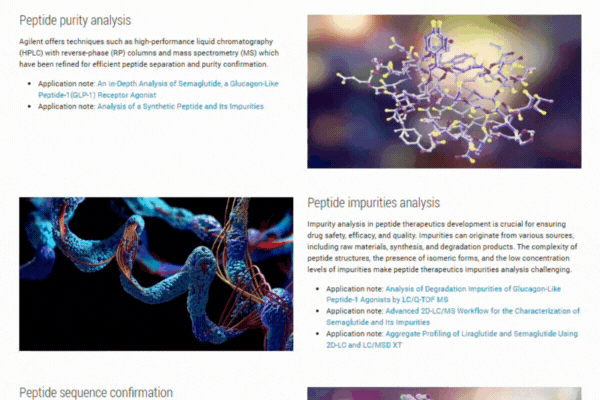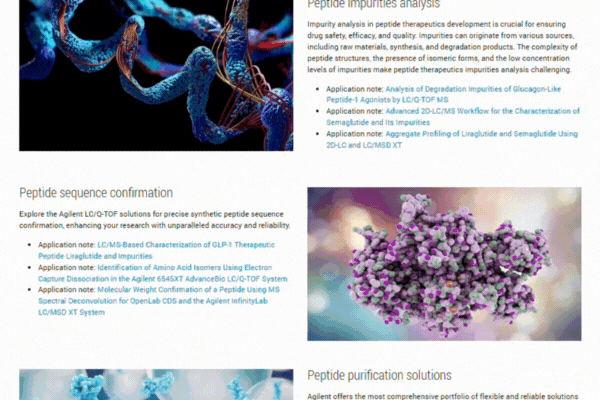
Explore our comprehensive resource hub on peptide therapeutics to enhance your research and development efforts

Download the infographic for end-to-end solutions and support to bridge every gap in your peptide characterizations.

Analysis and Purification of Synthetic Peptides by Liquid Chromatography

End-To-End Workflow Solutions for Therapeutic Peptides From research discovery to production QA/QC
Peptide Analysis and Purification: From Fundamentals to Advanced Techniques
Watch the on-demand webinar for a comprehensive overview of peptide analysis, designed for both emerging scientists and seasoned professionals.
Key focus areas include:
The session begins with an introduction to peptides and their unique analytical challenges. From there, we will delve into practical strategies for method development, covering essential parameters like mobile phase selection, ion pairing, pH, and temperature optimization. Additionally, we will discuss column screening, highlighting the benefits of various chemistries, particle sizes, and pore dimensions. Finally, we will explore preparative techniques and purification workflows, offering tips for achieving high purity and recovery.
Whether you're optimizing existing methods or developing new ones, this session will equip you with the tools to streamline peptide characterization and purification.
Key learning objectives:
For research use only. Not for use in diagnostic procedures.
DE-007734
Fill in the form below to be contacted by an Agilent representatives
Method validation is a critical step for biotherapeutic drug development. It ensures that the analytical method is suitable for its intended purpose, and that it meets regulatory requirements
Agilent AdvanceBio method validation kits simplify your method validation process. These convenient kits are equipped with columns designed to monitor critical quality attributes, guaranteeing the reproducibility and robustness of your method
Features: