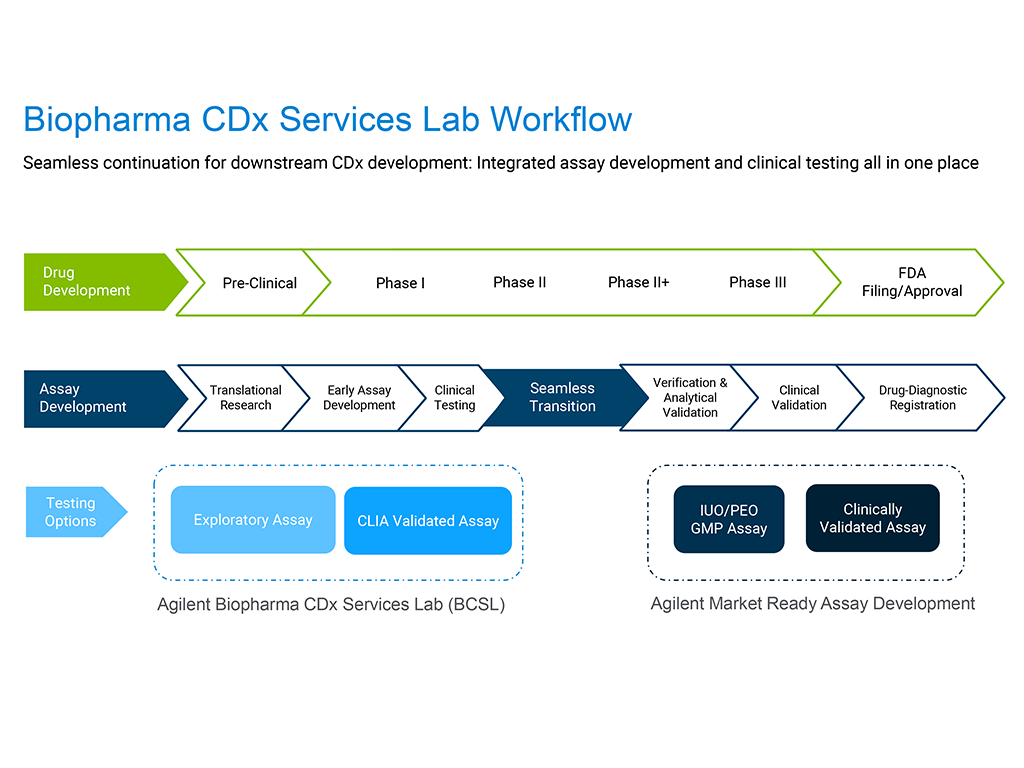
When developing highly complex assays or potential companion diagnostics (CDx), it is critical to have the utmost confidence in your early results– while also considering speed, budget, and a streamlined transition of your assays into later-phase clinical trials.
The Agilent Biopharma CDx Services Lab (BCSL) is a CLIA-certified facility offering fit-for-purpose assay and technology development services tailored for the needs of biopharmaceutical companies. Our expertise encompasses biomarker discovery and clinical trial assays for patient selection, with a seamless transition into CDx development, global regulatory approval and commercialization.
We offer a consultative and highly responsive project team to meet your early-phase clinical trial needs. Overall, within the BCSL, we are committed to accelerating your precision medicine program.
Agilent’s CDx experts will collaborate closely with you to optimize the assay development and sample testing plan that best fits your needs, including:
Exploratory Assays: Retrospective sample testing for signal searching, clone assessment, and other biomarker research
CLIA Validated Assays: Prospective sample testing for patient selection in early phase clinical trials, with a seamless transition to CDx development. All work is performed under Agilent’s robust Quality Management program and compliant to CLIA regulations.
In addition, for more complex biomarker programs such as those supporting bi- and tri-specific therapeutics or immuno-oncology combinations, you can leverage our multiplexing and/or digital pathology capabilities within the BCSL.
Our digital partner network can assist with machine learning algorithm development to optimize patient stratification, support multiplex IHC image analysis or low-level biomarker quantitation, and evaluate spatial biology biomarkers.
Comprehensive Solution: for a streamlined process from biomarker discovery to CDx commercialization, with an integrated assay development approach to avoid assay rework
Speed and cost: fit-for-purpose assays that match your clinical trial timeline, investment and risk-management needs
Quality assays: in-depth scientific, technical, and global regulatory expertise along with a robust Quality Management System
Multiplexing and digital pathology algorithm development capabilities: for complex biomarkers that may be difficult (or impossible) and time consuming to score manually, increasing accuracy and optimizing detection of a targeted population
With white-glove service and an exceptional track record in global regulatory approval and commercialization, Agilent is the clear choice to help seamlessly transition your assays from discovery to IVD development. You’ll accelerate your clinical trial timelines, manage your investment and regulatory risks, and gain a responsive partner throughout your CDx development journey.
Ready to learn more? Contact our BCSL team to discuss your project needs.
PR7001-2712
D0115645_1.00