Ready to talk to an an Agilent representative? Contact us here.
You can quickly and easily accomplish sample QC using Agilent automated electrophoresis solutions. These solutions help provide reliable sample quality control outcomes during the development and production of DNA- and RNA-based vaccines.
The diagram below shows a common RNA-based vaccine production workflow and recommended sample quality control steps (blue).
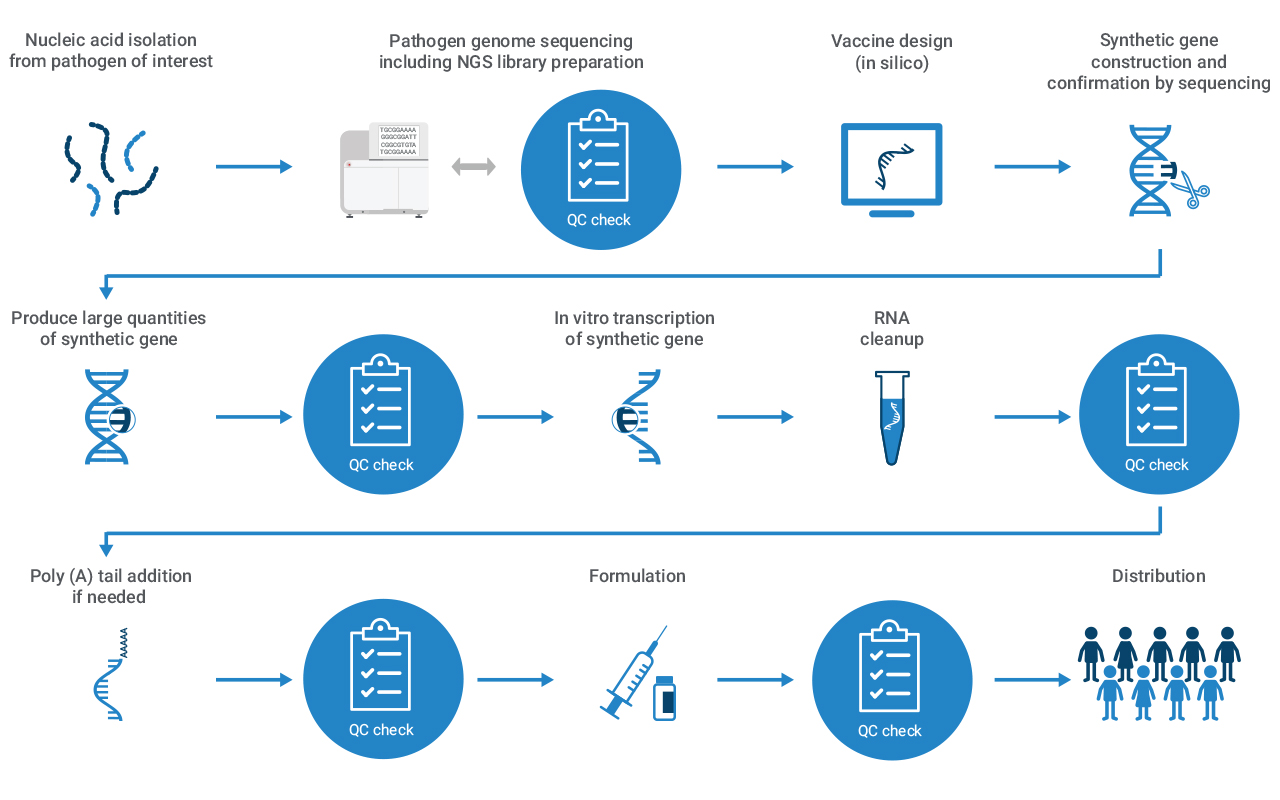
Nucleic acid-based vaccines, including those developed using in vitro transcribed (IVT) RNA, are manufactured with robust processes and are guided by good manufacturing practices (GMPs). GMPs include quality monitoring via sample quality control (QC) checks to detect poor-quality IVT RNA, which may be caused by insufficient PCR amplification, suboptimal plasmid linearization, incomplete transcription, DNA template contamination, or degraded RNA.
Automated electrophoresis solutions such as the Agilent Fragment Analyzer and TapeStation systems are easy to integrate into established workflows. These solutions help deliver confidence in sample quality assessments during critical vaccine research, development, and production steps.
Learn more about Agilent products for vaccine research and development in the interactive infographic.
This video shows how Agilent employees actively supported global pharmaceutical companies' COVID-19 vaccine production during the pandemic. In particular, Agilent's smooth cross-organizational collaboration with R&D, marketing, manufacturing, and logistics enabled quick fulfillment of urgent vaccine manufacturer requirements, supported by Agilent automated electrophoresis systems for reliable quality control of IVT RNA samples worldwide.