






Reliable QC. Informed decisions.
Knowing the quality of your nucleic acid samples throughout your vaccine development procedure is critical to make sure final products are effective and reliable. Accurate sample quality control (QC) enables you to assess the integrity of your samples and produce high-quality results.
Explore the QC steps at critical stages of the in vitro transcribed (IVT) mRNA vaccine development workflow in this infographic and discover how various Agilent solutions can enhance your sample quality assessment.



















Nucleic Acid
Isolation

Sequencing

DNA Template
Preparation

In Vitro
Transcription

RNA Quality
Check

Poly(A) Tail
Analysis

Formulation

Final Product


Nucleic Acid Isolation from Pathogen of Interest

Isolate nucleic acids after identifying the target pathogen.


Nucleic acid isolation of the pathogen is the first step in the development of a novel vaccine. The Absolutely Total RNA Purification Kits include the reagents needed for easy, fast, and highly sensitive DNA and RNA extraction and purification from tissue, cell samples or even coming from highly contaminated matrices such as wastewater. Learn More
 Quality control check
Quality control check
Assess nucleic acid integrity, purity, and quantity with Agilent automated electrophoresis systems to get accurate and reliable data for the subsequent sequencing experiment. Sample QC helps you minimize analytical errors in the NGS workflow.
Discover solutions



Pathogen Genome Sequencing

Perform library preparation, target enrichment, and sequencing to characterize the complete pathogen genome.



Agilent community designs offers NGS designs targeting many pathogens, including bacteria and viruses. The designs are for deep sequencing of pathogen genomes, and they enable target enrichment of pathogen sequences from human samples for infectious disease research. Learn more
 Quality control check
Quality control check
Assess the input sample quality and concentration to determine the optimal protocol. Evaluate intermediate products to ensure proper library construction or size distribution and check the quality of the final library prior to NGS. Sample QC using automated electrophoresis enables laboratories to conserve valuable time and resources.
Download white paper



DNA Template Preparation

After successful in silico vaccine design and synthetic gene construction, prepare DNA starting material from linearized plasmids or PCR amplification.
 Quality control check
Quality control check
Perform plasmid restriction digest screening or test the purity and concentration of the PCR products. Agilent automated electrophoresis systems deliver objective, reproducible data for DNA fragment sizing from low- to high-throughput applications.
Read application note



In Vitro Transcription of Synthetic Gene

Generate mRNA molecules from a DNA strand using RNA polymerase and nucleotides.
The RNAMaxx high yield transcription kit produces large quantities of RNA quickly and easily from a DNA template. Just 1 µg of template can yield 80 to 100 µg or more of RNA transcript in a short incubation. This is 10 to 30 times the yield produced by any conventional in vitro transcription reaction. The kit uses T7 RNA polymerase to produce high yields of RNA in just two hours. Learn more



RNA Quality Check

Purify RNA products and remove unincorporated nucleotides after in vitro transcription (IVT) is completed.
 Quality control check
Quality control check
Optimize in vitro transcription (IVT) reactions to ensure the correct and complete product is manufactured with acceptable purity. The quality of IVT RNA fragments can be assessed using the Agilent Fragment Analyzer and TapeStation systems. This analysis detects incompletely transcribed and degraded RNA products enabling optimization of the IVT reaction.
Download white paper



Poly(A) Tail Analysis

To help stabilize RNA molecules and minimize in vivo degradation, the addition of Poly(A) tails can be added to the transcript as part of the IVT RNA template or by a polyadenylase post transcription.
 Quality control check
Quality control check
Assess the presence of the Poly(A) tail to ensure proper product stability. Agilent Fragment Analyzer systems are used to approximate the length of the Poly(A) tail.
Explore the application



Formulation

Maximize product stability, enable efficient distribution, and support clinical product delivery requirements with a carefully designed vaccine formulation.
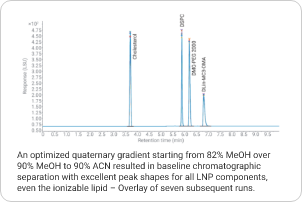
Lipid nanoparticles (LNPs) have emerged as promising delivery vehicles for nucleic acids in the pharmaceutical industry. To ensure safety and efficacy of the final product, LNPs need analytical characterization of composition, ratio, and degradation. The 1290 Infinity II Bio LC and 1290 Infinity II ELSD enable universal detection of the lipid components lacking a UV chromophore. Learn more





Final Product

Perform final QC checks on each lot to ensure product quality, and safety.
 Quality control check
Quality control check
Agilent Fragment Analyzer systems are well-suited for IVT mRNA purity analysis. The purity of the final product and the degraded product can be detected. Vaccine lots that pass QC testing are now ready for distribution to destinations for further on-site delivery.
Discover the solutions

For Research Use Only. Not for use in diagnostic procedures.
PR7001-1211