ctDx FIRST is an FDA approved companion diagnostic to KRAZATI™ (adagrasib) for the detection of KRAS G12C in non-small cell lung cancer (NSCLC) and provides tumor mutation profiling for single nucleotide variants (SNVs) and deletions in the EGFR gene for use by qualified health care professionals in accordance with professional guidelines.
*CLIA-validated, not FDA approved
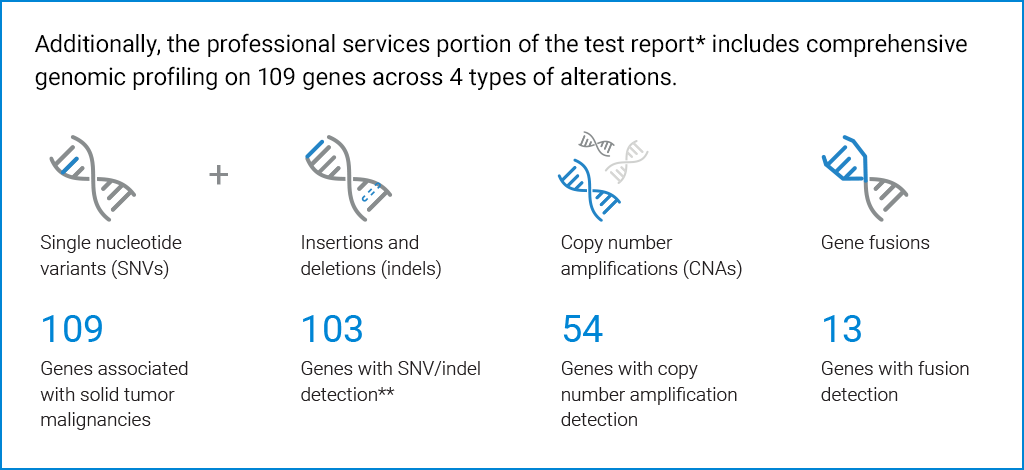
**64% of genes have full coding sequence (CDS) coverage
Intended Use:
The Agilent Resolution ctDx FIRST assay is a qualitative next generation sequencing-based, in vitro diagnostic test that uses targeted hybrid-capture sequencing technology to detect and report single nucleotide variants (SNVs) and deletions in two genes. The Agilent Resolution ctDx FIRST assay utilizes circulating cell-free DNA (cfDNA) isolated from plasma of peripheral whole blood collected in Streck Cell-Free DNA Blood Collection Tubes (BCTs). The test is intended as a companion diagnostic to identify non-small cell lung cancer (NSCLC) patients with KRAS G12C mutations who may benefit from treatment with KRAZATI™ (adagrasib), in accordance with the approved therapeutic labeling. A negative result from a plasma specimen does not assure that the patient’s tumor is negative for genomic findings. Patients with NSCLC who are negative for the KRAS G12C biomarker should be reflexed to tissue biopsy testing using an FDA-approved tumor tissue test, if feasible. Additionally, the test is intended to provide tumor mutation profiling for SNVs and deletions in the EGFR gene for use by qualified health care professionals in accordance with professional guidelines in oncology for patients with NSCLC. The test is for use with patients previously diagnosed with NSCLC and in conjunction with other laboratory and clinical findings. Genomic findings other than KRAS G12C are not prescriptive or conclusive for labeled use of any specific therapeutic product. The Agilent Resolution ctDx FIRST assay is a single-site assay performed at Resolution Bioscience, Inc.
Reference: Agilent Resolution ctDx FIRST [CDx Technical Information]. Santa Clara, CA: Agilent Technologies, Inc.PR7001-0188