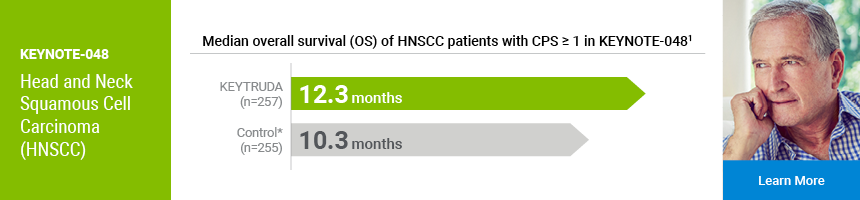
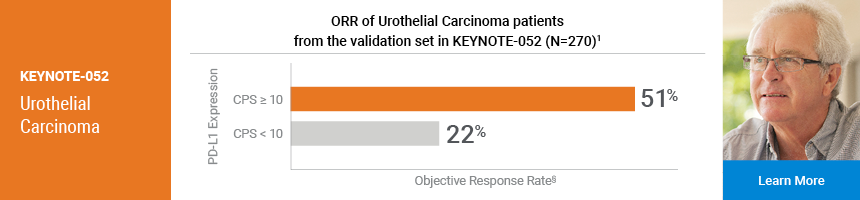
KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
References. 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2020. 2. Keytruda [Summary of Product Characteristics]. European Medicines Agency; 2020. 3. Emancipator, K.; Juco, J.; Huang, L.; Lunceford, J.; Cheng, J.; Ge, J.; Gause, C.; Swaby, R. F. Choice of PD-L1 Scoring and Cutoff in Head and Neck Squamous Cell Carcinoma (HNSCC) Trials of Pembrolizumab. USCAP 2020 Annual Meeting. 4. Plimack, E. R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L. Q. M.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R. F.; Odonnell, P. H. Safety and Activity of Pembrolizumab in Patients with Locally Advanced or Metastatic Urothelial Cancer (KEYNOTE-012): a Non-Randomised, Open-Label, Phase 1b Study. The Lancet Oncology 2017, 18 (2), 212–220. 5. Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Kulangara, K. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non–Small-Cell Lung Cancer. Applied Immunohistochemistry & Molecular Morphology 2016, 24 (6), 392–397.
For countries outside of the European Union, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.